Hatching chicken eggs at home can be a very rewarding experience. From collecting the eggs to seeing the chicks come out of their shells, it’s a fun process for people who keep chickens as pets. However, there are some important things you need to do in order for the eggs to hatch successfully. One of the most important is incubator temperature. Keeping the temperature just right is very important for the embryos to grow normally and hatch.
What temperature should you use to hatch chicken eggs? We’ll tell you everything you need to know in this detailed guide. We’ll talk about the best temperature range, how temperature affects embryo development, how to keep an eye on and change the temperature of an incubator, and a lot more. Whether you’re hatching eggs for the first time or want to improve your process, this guide has the temperature information you need to get them to hatch.
What Is The Ideal Temperature Range For Incubating Chicken Eggs?
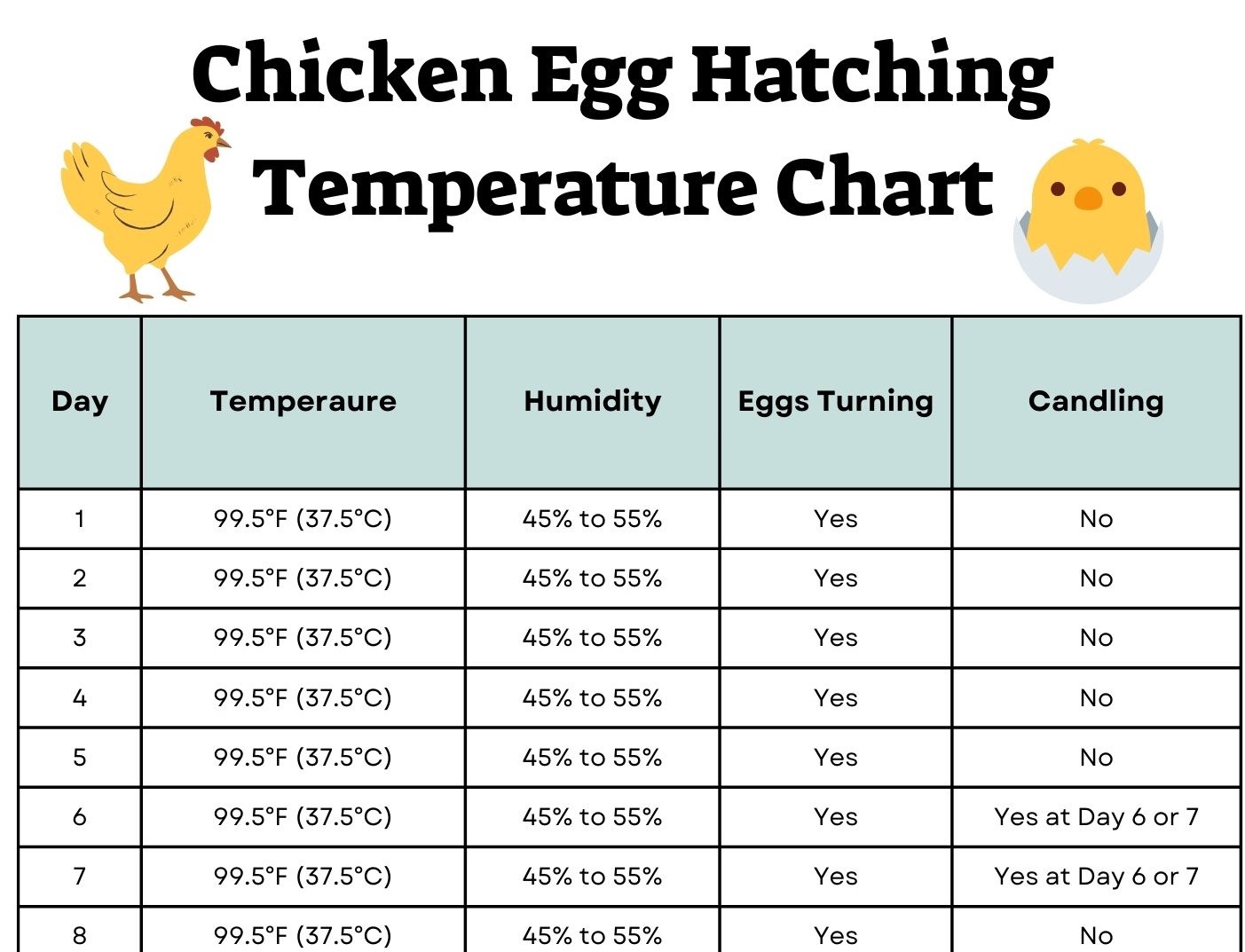
995°F to 1005°F (37.5°C to 38.1°C) is the best temperature range for incubating chicken eggs. Most people who hatch chicken eggs aim for 100°F (37.8°C), which is similar to the body heat that a broody hen gives to developing eggs.
Maintaining temperatures in this range during the 21-day incubation period creates the ideal setting for embryos to grow normally. Temperatures above or below this range can cause problems, such as deformities or death in embryos. That’s why consistency within the ideal range is so important.
Why Is Temperature So Critical For Chicken Egg Incubation?
Incubation temperature significantly impacts the speed and success of embryo development. The key processes of cell division and organ formation are highly temperature dependent.
If incubator temperatures are too low embryonic development will be slowed. Cell division and growth happens at suboptimal rates. This can result in weakened underdeveloped chicks that may not survive hatching.
Conversely, if incubation temperatures are too high, development will be accelerated. While this may seem beneficial, overly rapid development often leads to physical deformities, organ dysfunction, and death of embryos.
The ideal 99.5°F to 100.5°F range provides just the right amount of heat for embryos to undergo normal development with properly timed crucial processes. Maintaining this consistent temperature minimizes anomalies and maximizes hatch rates.
Are There Temperature Variations At Different Incubation Stages?
While the overall ideal temperature target remains 99.5°F to 100.5°F (37.5°C to 38.1°C) through most of incubation, there are a couple recommended variations:
-
Days 1-18: Maintain a steady, consistent temperature of 100°F (37.8°C)
-
Days 19-21: Slightly lower the temperature to 99°F (37.2°C).
This controlled temperature reduction in the last few days before hatching helps stimulate the chicks to get positioned for hatching. The drop in temperature triggers them to start pecking through their shells.
How To Monitor And Adjust Incubator Temperature
To maintain proper incubation temperatures, you need to regularly monitor and make adjustments as needed. Here are some tips:
-
Invest in reliable thermometers – a digital thermometer with a probe is ideal. Use multiple thermometers to cross-check accuracy.
-
Check incubator temperatures at least twice daily, ideally more. Make a log to record the readings.
-
Make small, gradual temperature adjustments if needed to maintain ideal range. Don’t make drastic changes.
-
Calibrate thermometers before use to ensure accuracy.
-
Place incubator away from windows, vents, direct sunlight, etc. to avoid temperature fluctuations.
-
Minimize opening the incubator to prevent heat loss.
With diligent monitoring and incremental tweaking of temperature as required, you can maintain an optimal and stable incubation temperature for great results hatching eggs.
How Does Humidity Factor Into Incubation Temperature?
While temperature gets most of the focus when it comes to incubating eggs, humidity also plays a critical role. The ideal humidity levels for chicken egg incubation are:
-
50-55% for days 1-18
-
65-70% for days 19-21 (lockdown period)
Humidity works in conjunction with temperature to ensure proper moisture levels and embryo development. Low humidity can cause the egg contents and membranes to dry out, while high humidity leaves too much moisture in the egg. Both scenarios can lead to hatching issues.
Monitoring both temperature and humidity helps identify if adjustments need to be made to optimize the incubation environment. The two factors work hand in hand for success.
Tips For Choosing An Incubator With Reliable Temperature Control
To properly regulate temperature when incubating eggs, you need an incubator that provides consistent, uniform heat. Here are some tips for selecting one with excellent temperature control:
-
Choose an incubator with automatic thermostat regulation to minimize temperature fluctuations.
-
Seek out incubators with good insulation to maintain interior temperatures. Avoid cheap styrofoam models.
-
Look for incubators with circulating fans to distribute heat evenly, especially for large capacity models.
-
Consider an incubator with an alarm system that alerts you if the temperature goes out of range.
-
Select an incubator with a built in, high-quality thermometer to avoid relying on separate thermometers.
-
When possible, invest in an incubator with a digital display that makes monitoring temperatures convenient.
While more advanced incubator models may cost more upfront, they often provide far better results by optimizing the all-important incubation temperature.
How Altitude Impacts Ideal Incubation Temperatures
An interesting factor that can influence incubation temperature requirements is altitude. Locations at higher elevations above sea level have lower air pressure. This affects heat distribution in incubators.
As a general guideline, for each 500 feet of elevation gain, incubator temperatures may need to be increased by around 1°F to compensate for the drop in air pressure.
So if you lived at 2500 feet above sea level, you might set your incubator target at 100.5°F rather than 100°F. Check with your incubator manufacturer for any altitude adjustments specific to the model you are using.
Common Temperature-Related Incubation Problems
Being aware of some common temperature-associated incubation issues can help you troubleshoot problems:
-
Early hatching – Incubator running hot, temperatures too high
-
Late hatching – Incubator running cool, temperatures too low
-
Stuck chicks – Temperature fluctuations during hatch window
-
Deformed/dead embryos – Excessively high temperatures
-
Large air cells – Low humidity along with high temperatures
Monitoring your incubation temperatures closely and making timely adjustments will help avoid these problems and lead to better hatch rates.
Maintaining Proper Temperatures During Hatching
Once eggs begin externally pipping as chicks start to emerge, some temperature rise is normal and expected. The eggs and incubator may heat up a degree or two due to chick activity at hatching. As long as temperatures don’t exceed 103°F for an extended time, this is fine and no adjustment is needed.
Providing Correct Brooder Temperatures Post-Hatch
The temperature needs of newly hatched chicks differ from incubation temperatures. Here are the recommended brooder temperatures:
-
Week 1: 95°F (35°C)
-
Decrease 5°F (3°C) per week until ambient temperatures are reached
Gradual temperature reduction allows the maturing chicks to adjust to lower temperatures as they feather out and build body heat.
The Takeaway On Incubation Temperatures
The key points to remember when considering what temperature to use for incubating chicken eggs are:
-
Ideal range is 99.5°F – 100.5°F (37.5°C – 38.1°C)
-
Consistent temperatures are crucial – minimize fluctuations
-
Monitor temperatures frequently and make incremental adjustments as needed
-
Invest in a quality incubator with excellent temperature control
-
Consider both temperature and humidity for optimal incubation
Follow these tips, and you’ll be rewarded with healthy chicks hatching right on schedule! Let us know if you have any other questions arise as you embark on your egg hatching journey.
Incubation Temperature and Post-Hatching Adaptive Response
The effect of temperature manipulation during the embryogenesis on post-hatch adaptive stress response may be explained by mRNA expression of genes involving stress response, thermoregulatory and metabolic programming (Loyau et al. , 2016). Comparing thermally manipulated and control chicks under heat stress conditions showed that 759 genes were differently expressed (Loyau et al. , 2016). There are proteins called heat shock proteins (Hsp) that help cells deal with heat stress and keep structural proteins intact. Al-Zghoul. (2018) found an increase in Hsp70 expression in heat-stressed chickens exposed to incubation temperatures of 38. 5–39. 5°C for 18 h from ED12 to 18 of embryogenesis. Because heat stress causes inhibition of protein synthesis, an increase in Hsp70 mRNA expression in heat-stressed chickens would be associated with an improvement in protecting cell integrity in chickens (Al-Zghoul et al. , 2013). It was also shown that genes that code for parts of the CRF signaling pathway change how they are expressed in the hypothalamus of chicks that have been heated or cooled. This shows that heating or cooling the hypothalamus causes epigenetic changes (David et al. , 2019).
The studies also attempt to evaluate lower incubation temperature and its effect on adaptive response. Shinder et al. (2011) found that between ED18 and 19, a short (30 min) exposure to 15°C cold did not affect the ability to hatch, but it did speed up growth and lower the risk of ascites. Similarly, 6 h/d low temperature (36. The temperature increase (6°C) from ED10 to 18 made broilers gain weight and become better at handling cold. This led to long-term changes in their antioxidant defenses and energy metabolism (Aksit et al. , 2013; Loyau et al. , 2014). When embryos and day-old chicks were kept at 36°C for 36 hours, changes were seen in the levels of antioxidants and fatty acids in their brain and liver tissues. 6°C, 6 h/d from ED10 to 18. These changes may be accepted as coordinated adaptive reactions of chicks (Yalcin et al. , 2012b).
Several studies have also shown that high temperatures promote muscle development and myoblast proliferation in day-old chicks. Piestun et al. (2009) showed that during late-term embryogenesis (ED16 to 18), high incubation temperature (39.5°C for 3 or 6 h daily) increased muscle insulin-like growth factor I (IGF-I), which enhanced muscle cell proliferation and differentiation, and myofibers diameter. However, as the study was ended at post-hatch d 13, if muscle development was affected at slaughter age is unknown. Our recent finding (Yalcin et al., 2021) suggested that exposing Ross308 and Cobb embryos to 38.8°C between ED10 and 14 resulted in heavier body weight and higher insulin-like factor-1 (IGF-I) expression, and larger fiber area in breast muscle of broiler chickens at slaughter age. However, breast muscle properties of strains, i.e., expression of vascular endothelial growth factor-A and myogenin, carcass part yields, pH24, and water holding capacity of strains responded differently to temperature manipulation (Yalcin et al., 2021). This result supports further evidence that the effect of thermal manipulation is strongly related to the strain.
In conclusion, the studies showed that the effect of incubation temperature during embryonic development is undoubtedly crucial for adaptive stress response. Incubation temperature could program the chick to construct traits in adaptation to a post-hatching temperature environment. This response may be explained by the imprinted epigenetic changes in the hypothalamus that trigger a response when the chickens are again exposed to high or low temperatures (David et al., 2019). The studies tell us that interaction among timing, duration, and temperature shape embryo development and adaptive stress response. Indeed, Wilsterman et al. (2015) showed that exposure of embryos to slightly higher temperatures either early, late, or whole incubation period had an impact on the pattern of glucocorticoid release, however, the specific response of chicks and broilers varied with the timing. Currently, it is unclear how the sensitive period and temperature interact with the other environmental factors in the incubator and maternal factors (strain, breeder age, egg composition, and egg quality). Nevertheless, during the second half of incubation, the embryo may be more sensitive to temperature manipulation signals to have a long-lasting post-hatch effect. Further studies are needed to understand the effect of epigenetic modifications during embryonic development, their molecular mechanisms underlying these changes, and their long-term effects.
Role of light stimulation during incubation on embryonic muscle growth and stress. HPA, Hypothalamus-Pituitary-Adrenal; GHRH, Growth Hormone-Releasing Hormone; CRH, Corticotrophin Releasing-Hormone; ACTH, Adrenocorticotropic Hormone; TRH, Thyrotropin-Releasing Hormone; GH, Growth Hormone; IGF-1, Insulin-like Growth Factor-1.
Therefore, photostimulation at early or late periods of embryonic development has been investigated in the studies to see if lighted incubation affected embryo development and hatching performance. However, not only the critical periods but also the duration of photostimulation per day and characteristics of light including intensity, color (wavelength), and color temperature of light are important. In this part of the paper, we review the effect of light provision during incubation on embryo development and post-hatching growth, and the post-hatching adaptive response of chicken, taking into account timing, duration, color, and intensity.
Effects of Light on Embryo Development and Post-Hatching Growth
Chicken embryos can detect color differences. Several studies have been conducted to investigate the effect of light color on embryonic development and post-hatching growth. As compared with blue light and dark incubation conditions, continuous green light during the incubation enhances the post-hatch body weight of male broilers, improves the feed conversion ratio, increases the satellite cell mitotic activity of the pectoral muscle with upregulation of MyoD, myogenin, and myostatin mRNA expression in late embryos and newly hatched chicks, and muscle growth with no noticeable changes in chemical composition and meat quality characteristics (Zhang et al., 2012, 2014). Providing green light intermittently (light/dark cycles of 15 min) during incubation also increases hypothalamic expression of growth hormone-releasing hormone (GHRH), liver growth hormone receptor (GHR), levels (Dishon et al., 2017). Dishon et al. (2021) compared intermittent green light stimulation throughout the incubation (ED0-21) with different stimulation periods starting from ED15, 16, and 18 of incubation and observed a higher expression of the somatotropic axis genes in all lighting treatments than in dark incubation. They suggested that photostimulation of embryos only last 3 days of incubation would be enough to stimulate the somatotropic axis since photostimulation of embryos from ED18 to hatch resulted in similar expression levels of hypothalamic GHRH, liver GHR, and IGF-1 genes and GH plasma levels to the positive control group (lighted from E0-21). These findings deserve to be investigated further to establish a clear conclusion regarding the critical period for the growth-stimulating effect of green light on broiler embryos.
The pineal gland of the chick embryo shows a selective sensitivity to different wavelengths (color) of the light spectrum. Drozdova et al. (2019) found a higher biosynthesis of pineal melatonin during scotophase under red (632 nm) and white (a peak wavelength of 448 nm) lighting compared to green (517 nm) and blue (463 nm). Further research from the same group showed that red-lighted incubation resulted in higher body weights in broiler chicks during the post-hatch rapid growth phase (from 18 to 21 days) compared to blue light (Drozdova et al., 2021). Although there is not much information regarding the effect of red light on the somatotropic axis, increased growth of chicks incubated under red light may be related to the early entrainment of melatonin rhythms. Not only the light color but also the color temperature of polychromatic light would be important. The cool white LED (5,000 K) containing more blue wavelength could improve weight gain and reduce stress and fear responses of broilers as compared to warm white LED (2,700 K) (Archer, 2018). However, it was shown that incubation in warm and cold white light did not significantly influence embryonic melatonin biosynthesis in the pineal, T3, T4, corticosterone hormone levels in the blood and immune system-related genes, presenilin-1, and avian betadefensin1, in the duodenum and bursa Fabricius (Drozdova et al., 2020). The authors concluded that selective effects of distinct wavelengths on embryonic and post-embryonic development might be more profound than the effects of change in the color temperature of polychromatic light.
Limited research is available regarding the effect of lighting during the incubation on bone growth and leg health, and the results are not consistent. An improvement in leg health of broilers was found using a 16L:8D (van der Pol et al., 2017) or 12L:12D (van der Pol et al., 2019) at 500 lux white LED lighting compared to continuous light or dark incubation conditions. However, in a recent study, Güz et al. (2021) did not find any significant effect of green LED light on tibia bone parameters when they used a 16L:8D photoschedule. It is necessary to clearly reveal whether the light color will affect bone development.
The intensity of light has also been the subject of interest. In a recent study, Yu et al. (2018) reported that 50 lux intensity using green LED light (16L:8D) increased chick length, weight, hatchability, testosterone, and T4 hormone levels and reduced hatching time, i.e., an earlier peak of 12 h, in newly hatched chicks compared to 150 and 300 lux. However, the transmission of light into the eggs significantly varies with the level of pigmentation and the conductance of eggshells (Shafey et al., 2002). Shafey et al. (2002) compared the spectral absorption rate of pigmented and non-pigmented eggshells over the wavelength range between 200 to 1,100 nm. Brown pigmented eggs had a max absorption rate of 99.96% for the near-ultraviolet region (wavelength ≤380 nm) of the light spectrum, which was higher than the absorption rate of 99.88% for long wavelengths, about 1,075 nm at the near-infrared region. Shafey et al. (2005) also compared two high intensities changing between 1,430–2,080 and 900–1,380 lux using a green fluorescent light source and different pigmentation levels of brown eggshells. They reported that higher intensity resulted in higher embryo mortality and decreased hatchability in light pigmented brown eggshells while there was no negative effect for dark drown eggshells (Shafey et al., 2005). Yu et al. (2016) confirmed that eggshell pigmentation and the region of the eggshell determine the transmission of visible wavelength (380–780 nm) into the egg. These findings support the hypothesis that the evolution of eggshell pigmentation for selective transmission of different wavelengths into eggs is based on preventing the negative effects of ultraviolet and infrared light (Maurer et al., 2011). Maurer et al. (2015) supplied further evidence from wild birds and concluded “avian eggshell properties, including eggshell structure and pigmentation, which are consistent with an evolutionary pressure to both enhance and protect embryo development”. Huth and Archer (2015) investigated the effect of eggshell pigmentation on the spectrum of light filtered by eggshell. They reported that the spectrum of light filtered by white eggshells was quite similar to unfiltered light; however brown eggshells produced a redder spectrum as evidence of higher transmission of long wavelengths into eggs. Recently Güz et al. (2021) observed that a green LED light source with a peak light spectrum of 522 nm yielded a 536 nm peak in the light spectrum after passing through the eggshell of broiler breeder eggs showing that pigmented eggshell may change wavelength reach into the egg. It is clear that the wavelength and intensity of light that reach the embryo are limited by eggshell properties. It should also be considered that the lux unit is based on human spectral sensitivity. Bird’s spectral sensitivity to short (400–480) and long (580–700) wavelengths is higher than humans due to their additional cone type of photoreceptor cells (Lewis and Morris, 2006). Therefore, eggshell properties, wavelength, and intensity of light both outside and inside of the egg should be taken into account in future studies to have a finely tuned lighting program for broiler embryos.
The available data presented above show that there is no accepted standard lighting procedure in the incubator until now. It can be concluded that green light is the most effective for stimulating growth and improving muscle development. However, homogenous intensity should be kept with lower intensities inside the incubator. Red light may have a more profound effect on innate immunity while green light may affect the immune and inflammation, and stress response of broiler chicks. However, further research is needed underlying mechanism for early immune programming and interactions between the light source and embryo development.
Incubating Chickens is SUPER Easy!
FAQ
What is the best temperature for hatching eggs?
In the incubator, put the eggs in the egg tray so that the wider end faces up and the narrow end faces down. Set the temperature to 100. 5 degrees Fahrenheit with 50-55 percent humidity.
Is 75% humidity too high for hatching eggs?
Ideal Humidity Ranges for Different Eggs Chicken eggs: 35-55% during incubation, 65-75% during hatching. Duck eggs: 50-60% during incubation, 65-80% during hatching. Reptile eggs: Varies depending on the species, but generally between 60-70% is common.
How can you tell when a chicken egg is ready to hatch?
Chickens can hatch between 19–22 days after you put the eggs in the incubator. Signs to look for include the egg being cracked by the beak of the chick and chirping from them as they start to emerge.
What is the optimum temperature for incubation?
Incubation temperature ranging between 37 and 38°C (typically 37. 5–37. 8°C) optimizes hatchability. However, the temperature inside the egg called “embryo temperature” is not equal to the incubator air temperature.
What are the optimal conditions for incubating and hatching chicken eggs?
The optimal conditions for incubating and hatching chicken eggs involve maintaining specific temperature and humidity levels. Here’s a summary of the optimal conditions: Incubation Temperature: The ideal temperature for incubating chicken eggs is around 37. 5 to 37. 8 degrees Celsius (99. 5 to 100 degrees Fahrenheit).
How long does it take for chicken eggs to hatch?
A: Chicken eggs hatch in about 21 days, but this can change depending on the breed and the conditions of the incubator. Q: Can I use LED bulbs in my incubator? A: Yes, LED bulbs are a suitable alternative to traditional incandescent bulbs and can provide the right spectrum of light for hatching eggs.
What temperature should a chicken egg be incubated at?
Research shows that the optimal temperature range for chicken egg incubation is 99 to 102 degrees Fahrenheit. Straying outside this range—even briefly—can result in developmental issues or failed hatching. Think of the embryo as a complex machine that’s programmed to operate at a certain temperature; deviating from the norm causes malfunctions.
