A fertilized chicken egg is a remarkable thing. In just 21 days, it can transform from a simple egg into a living, breathing chick! But how long can a fertilized egg actually survive before it must be incubated? Let’s take a closer look at the fascinating lifecycle of a chicken egg and the factors that affect its chances of hatching successfully.
The Magic 21 Days
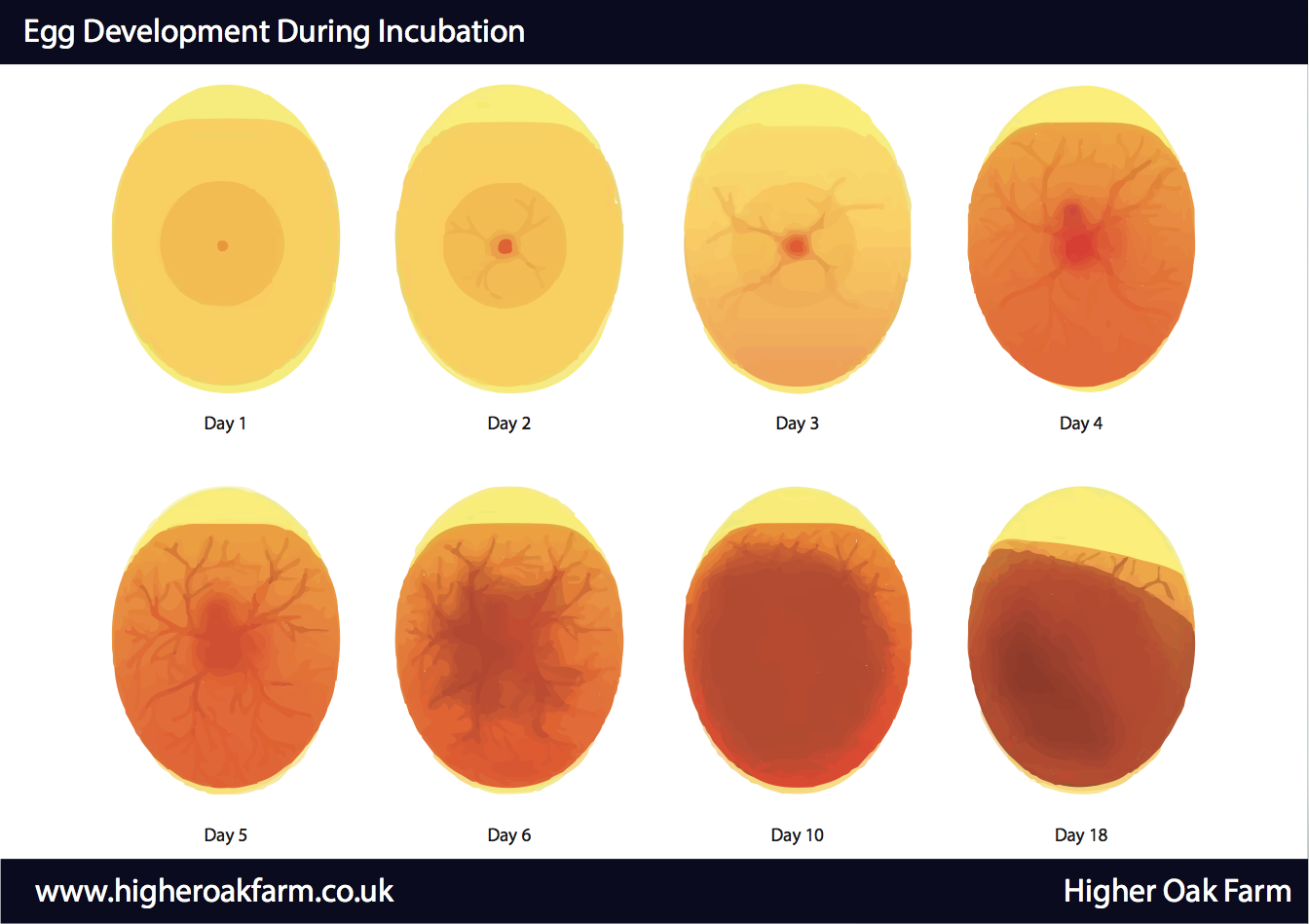
Usually, it takes 21 days for a chicken egg to hatch. During this time, the embryo grows inside the shell, going from a small disc to a fully formed chick that is ready to hatch and enter the world.
During the first week, the major organs like the heart, eyes, and central nervous system begin forming. By day 14, feathers and toenails start to grow. And in the final week, the chick gets into hatching position and prepares to peck through its calcium fortress and take its first gulps of air.
So 21 days is the norm. But is that also the longest time a fertilized egg can live?
Pushing the Limits
It turns out that fertilized chicken eggs can survive longer than 21 days outside an incubator, given the right conditions. While their hatchability declines over time, embryos have been known to successfully develop and hatch after 3-4 weeks of being unincubated.
Backyard chicken owners report hatching chicks from eggs stored for 4 weeks or even more! However, the chances of hatching significantly diminish as storage time increases past 10-14 days. By 6 weeks, few if any embryos could remain alive.
So while 21 days is the norm, chicken eggs can last unincubated for up to 35 days in exceptional circumstances But their likelihood of hatching steadily decreases the longer incubation is delayed.
Factors Affecting Egg Viability
What allows a fertilized egg to survive for weeks outside an incubator, defying the 21 day rule? And what causes others to perish more quickly? There are several key factors at play:
1. Storage Conditions
To stay viable for extended periods, eggs must be stored properly before incubation. Keeping them:
-
At cool temperatures between 55-68°F (13-20°C).
-
With the pointy end facing down to keep the air cell stable.
-
In an environment with around 70% humidity to prevent moisture loss.
-
Clean and free of cracks or damage.
If you follow these steps, the egg will stay healthy and the embryo will stay dormant but alive for weeks.
2. Egg Freshness
The fresher the egg, the longer it will keep. Eggs less than 7 days old have the highest hatchability rates. After 2 weeks, their fertility drops off significantly. Older eggs over 1 month should be candled to check for signs of life before incubating.
3. Breed Differences
Some chicken breeds, like Bantams, lay eggs that last longer than most because the yolks are denser and the shells are thicker. The hen’s genes affect how long her eggs will last when stored.
4. Temperature Fluctuations
Once incubated, chicken eggs are sensitive to overcooling or overheating. Temperatures deviating more than 1-2°F from 99.5°F during incubation can jeopardize development. Minor fluctuations are tolerated, but extremes will be fatal.
5. Disturbances and Shaking
Vigorous shaking, rotation, or repositioning of eggs once incubation begins can traumatize the embryos and lead to death. After a week of sitting undisturbed, the eggs must be turned gently to prevent sticking.
6. Infections and Contamination
Bacterial infections in the parent birds, like Salmonella, can infect the egg and cause early death of the embryo, especially after 2 weeks. Cracks in the shell also raise contamination risks. Both limit how long eggs survive.
The Takeaway
While 21 days is the standard incubation duration, chicken eggs can remain viable for up to 35 days given excellent storage conditions. However, their hatchability steadily declines the longer incubation is delayed past 10-14 days. Maximize their longevity by keeping eggs cool, pointed down, and humidified before incubating according to breed guidelines. With proper care, life can persist for weeks inside a dormant egg!
So if you chance upon a forgotten fertilized egg, don’t assume it’s too late. It may still hatch a healthy chick given the right care and environment. Just be sure to candle regularly for signs of life as you approach 4 weeks. With a little luck, this egg may defy the odds and become the chick that lasted!
MRI Analyses During Incubation
The eggs stored for 3 days (D3) or 10 days (D10) were incubated. After 11, 13 or 15 days (EID11, EID13, EID15), eggs were collected and refrigerated at 4°C for 1 h and 10 min at −20°C prior to analyses with 3 T (T) MRI scanner (Siemens Magnetom®, Verio, Erlangen, Germany). Such an egg cooling was necessary to anesthetise the chick and thus avoid movements of the embryos during MRI acquisition.
We used one radio frequency (RF) ‘loop’ coil, with an inner diameter of 7 cm, to analyse eggs independently. Each egg was inserted in the middle of the loop coil.
Two separate MRI sequences (T1: spin-lattice or longitudinal relaxation time and T2: spin-spin or transverse relaxation time) were performed on the whole brain, in order to get two distinct contrasts. The T1 3D and T2 3D were the Magnetisation Prepared Rapid Acquisition Gradient Echo (MPRAGE) and the Sampling Perfection with Application-optimised Contrasts (SPACE), respectively.
The acquisition parameters for these T1 and T2 anatomical analyses were as follows:
-T1 3D: repetition time (TR) = 1970 ms; echo time (TE) = 3. 34 ms; inversion time (TI) = 900 ms; flip angle = 9°; field-of-view (FOV): 81 * 81 mm2; matrix: 192 * 1922; and a slice thickness of 0. 4 mm resulting in a voxel size of 0. 42 * 0. 42 * 0. 40 mm3. A bandwidth of 150 Hz/Px and two number of excitations (NEX) producing an acquisition time of 9 min 29 s were used.
-T2 3D: TR = 1860 ms; TE = 140 ms; flip angle = 140°; bandwidth of 296 Hz/Px; and a turbo factor of 99. The inter echo space was 7. 38 ms. The FOV was 70 * 70 mm2. The matrix was 192 * 1922 and slice thickness was 0. 35 mm, which ended with a voxel size of 0. 36 * 0. 36 * 0. 35 mm3. The acquisition time was 8 min 57 s.
Volumes on the MRI s were estimated based on T1 s of the yolk and the albumen, and on the T2 s for the brain, eyes, yolk sac, allantoic fluid and embryo, as T2 contrast clearly allows distinction of water content between egg compartments (Table 1). At EID13 and EID15, the signals from the yolk sac and the embryo were mixed up because the tissue of the yolk sac, which is very dense and well-vascularized (Wong and Uni, 2021), looked like the tissue of the embryo and the two structures could not be told apart.
For volume estimation, the Digital Imaging and Communication in Medicine (DICOM) s were converted into the Neuroimaging Informatics Technology Initiative (NIfTI) format.
The NIfTI s were read with ITK Snap, which is a free, post-processing software generally used to segment 3D medical structures (Yushkevich et al. , 2006). The segmentation of “area growing” type was done automatically and then corrected manually.
After MRI analyses, eggs were weighed, embryos were removed from the eggs and decapitated and embryo weight was determined. Small pieces of the liver were collected and stored at −20°C for further analysis (molecular sexing).
Molecular sexing was performed as previously published with minor adjustments (He et al. , 2019). Small pieces of EID11, EID13 and EID15 embryo livers were lysed in 150 μl of lysis buffer containing 10% of chelating beads (Chelex 100), 0. 2% SDS, 10 mM Tris pH 8 and 0. 2 mg/ml Proteinase K). Samples were incubated for 3 hours at 55°C followed by a 15-min incubation at 95°C. Samples were then centrifuged for 3 min at room temperature at maximum speed with a Mini centrifuge 6K (ExtraGene, Taichung City, Taiwan). Supernatants were recovered and stored at −20°C until use. DNA lysate quantification was assessed by reading the 260 nm absorbance with a micro volume spectrophotometer (Nanodrop One Thermo Scientific, Wilmington United States). Embryo lysates were diluted ten times in nuclease free water and 1 μl of dilution was mixed on ice with primer SWIM (forward: 5′- GAGATCACGAACTCAACCAG -3′/reverse: 5′- CCAGACCTAATACGGTTTTACAG -3′), which is female specific and primer 12S (forward-5′ CTATAATCGATAATCCACGATTCA- 3′, reverse: 5′- CTTGACCTGTCTTATTAGCGAGG -3′) and Dream Taq PCR Master Mix (2X), according to the manufacturer’s recommendations (Thermo Fisher Scientific, Illkirch, France). Amplification by polymerase chain reaction (PCR) was performed using a thermocycler (Eppendorf, Montesson, France), as described previously (He et al. , 2019). PCR products were loaded on a 2% agarose gel containing 0. 01% gel Red in 1X TAE buffer, and separated by electrophoresis at 100 V. Gels were d using a Bio-Print r (Vilber Lourmat, Marne-la-Vallée, France). In female samples, there were two amplification products: one for the 12S gene (131 bp) and one for the SWIM gene (212 bp). In male samples, there was only one amplification product for the 12S gene.
All statistical analyses were performed using XLSTAT software (Data Analysis and Statistical Solution for Microsoft Excel, Addinsoft, Paris, France 2017). For most parameters, normality of the samples was not achieved (Shapiro–Wilk test). Thus, all statistical analyses (except for embryo weight) were performed using a Kruskal–Wallis test (p < 0. 05), followed by a pair comparison using a Mann–Whitney test (p < 0. 05), when required. For embryo weight, we used an ANOVA test.
Incubation Procedures and Sampling
We got fertilized eggs from broiler breeder hens that were 30 weeks old (ROSS 308, Boyé Accouvage, La Boissière en Gâtine, France). Eggs were all weighted and ten-egg batches of similar egg weight (56. 5 ± 0. 52 g) were formed. The egg weight in each batch ranged from 53 to 60. 4 g to illustrate natural egg weight heterogeneity. Only ten eggs (D0) were kept to be analyzed. The other eggs were put away in the Poultry Experimental Facility (PEAT) UE1295 (INRAE, F-37380 Nouzilly, France, DOI: 10 15454/1. 5572326250887292E12) in a dedicated room at 16°C, 80% RH for three (D3) or 10 days (D10). Ten eggs were collected at D0, D3 and D10 and were analysed by computed tomography, followed by the measurement of some egg quality parameters. The day before incubation, eggs were kept at room temperature (45% humidity) and then moved into a Bekoto B64-S egg incubator (Pont-Saint-Martin, France) that was set to 37 8°C, 55% RH (automatic turning every hour, large end of eggs on top). We checked D3 and D10 eggs with viable embryos using computed tomography after 11, 13, and 15 days of incubation (Embryonic incubation day 11, 13, and 15 or EID13, EID13, and EID15, respectively). Other eggs were chosen for MRI analyses. After getting CT and MRI scans, all the eggs were weighed and opened to get the embryos that had been killed by cutting off their heads. This experimental procedure meets the guidelines approved by the Institutional Animal Care and Use Committee (IACUC). Dead embryos were weighed and a small piece of the liver was collected, kept at −20°C for molecular sexing.
How Long Can an Egg Sit Before a Hen Starts Sitting on Them?
FAQ
How long is a fertilized chicken egg good for?
Prior to incubation, a fertilized egg can be stored for a maximum of 7 days in a cool room kept at a steady 55-60 degrees Fahrenheit (not in the refrigerator – it’s too cold!).
How to keep fertile eggs before incubation?
Before laying eggs, make sure they are stored properly by keeping them in a cool, dry place that is between 55 and 60°C (13 to 16°C). The humidity should be between 70 and 75%. Turn the eggs daily, and store them pointy end down.
How to tell if a fertilized egg is still alive?
If you’re not sure if the embryo is still alive, put the egg back in the incubator and check again more quickly. A second test can be made after 14 to 16 days of incubation. If the embryo is living, only one or two small light spaces filled with blood vessels can be seen, and the chick may be observed moving.
Will fertile eggs hatch if they get cold?
Don’t put an egg you want to hatch in the fridge–the cold will kill the embryo. Hatching eggs should be clean and stored at 55–65 F and 70–80% humidity. Even then, hatchability fall off quickly after 10 days in storage.
